|
BP-292E
PULP AND PAPER:
Prepared by: TABLE
OF CONTENTS THE PULP AND PAPER INDUSTRY AS A POLLUTION SOURCE A. The Structure of Woody Materials IDENTIFICATION OF COMPOUNDS HAZARDOUS TO HUMAN HEALTH POLLUTION ABATEMENT BY THE PULP AND PAPER INDUSTRY B. End-of-Pipe Biological Treatment Processes C. Pollution Abatement – A Case Study
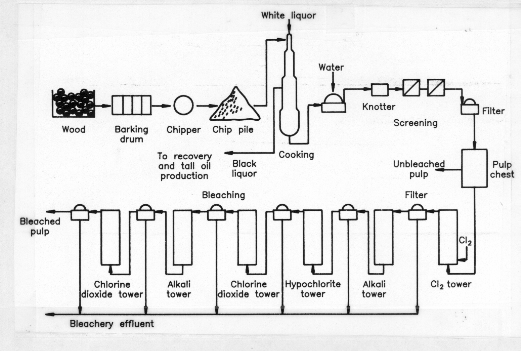
PULP AND PAPER: The manufacture of pulp and paper is a resource-intensive industry that consumes large amounts of energy, water, and trees. It is a mature industry that is innovative and adapts rapidly to technological change in order to remain competitive in the international market. The emergence of this technology as a major industry in Northern Europe and North America took place during the latter half of the nineteenth century, a time when there was little thought given to the possible detrimental effects of industrial pollution on the environment or human health. In the United States, pulp and paper mills are now considered the nation’s third largest polluter.(1) In Canada, it has been estimated that this industry is responsible for 50% of all the waste dumped into the nation’s waters(2) and also accounts for approximately 5.6% of the common air contaminants from known industrial sources.(3) Pollution from the pulp and paper industry is both a tradition in Canada, and a consequence of international economic reality. The technologies exist for substantially reducing emissions, particularly those that are a risk to human health. However, pulp and paper mills represent capital expenditures in the high hundreds of millions of dollars, and retrofitting a mill with new process technologies for pollution abatement regularly costs additional hundreds of millions of dollars. Such huge expenditures put pulp and paper companies at a disadvantage with competitors in countries without stringent effluent controls The implementation of environmental protection will have to proceed in a manner that sustains the economic health of the pulp and paper industry, yet ensures human health and environmental quality. To tackle this problem in a cost-effective manner it is necessary to understand the nature of the problem, and identify the specific compounds that endanger human health and environmental quality. It is then possible to choose and install new process technologies to prevent the production of toxic effluents. Alternatively, depending upon economic circumstances and the age of the mill, it may be more cost-effective to add an end-of-pipe waste water treatment system. There is a large degree of variation in the pulping and bleach plant technologies used in mills. Accordingly, the solution for one mill cannot be applied to the environmental problems of the whole industry. Egyptian papyrus and Chinese silk cloths were the first writing materials. The first true paper, prepared from fibrous cellulose-containing plant material, was manufactured in China in the year 105 A.D. At that time, Chinese clothing was made largely from China grass (Boehmeria nivea), a common fibrous plant. Worn-out clothing was collected by rag merchants and sold to paper makers, who recycled the cellulose content into paper.(4) The technology of paper-making slowly crossed the Arab world, and by the year 1150 made its way to Europe through Moorish Spain. From 1150 to the middle of the 19th century the feedstock for paper-making remained recycled cellulosic materials, such as rags, rope, fish nets, and burlap. European clothes were made primarily of either linen or wool. Flax, from which lien is made, was therefore the dominant source of European paper for centuries.(5) The flax plant Linum usitatissimum) grows to 1.2 metres in height, and produces woody fibres, which are much longer than real wood fibres and unlike them are easily separated, very strong, and supple-soft. These qualities are valued for the production of specialty papers that are soft, yet very strong. Today, most of the world’s bank notes are printed upon a high-value flax paper produced in Canada. The first paper mill in Lower Canada was established in 1803, in the village of St. Andrews, near present-day Lachute, P.Q. By 1820, a number of mills had begun operation across Upper and Lower Canada. Until Confederation, these mills were generally small, and depended upon linen and cotton rags and recycled rope, jute cloth, flax wastes and straw, for their raw feedstock. In Georgetown, Ontario, the Barber paper mill installed straw boilers in 1861, and made paper from straw from local farms. At this time, all the cellulosic substrates used in paper-making were either already well processed or raw materials from which cellulose fibres could be extracted easily. Wood was not used as a substrate, as the technology and equipment had not been developed to chisel and grind wood down inexpensively into a size that could be used in the established paper-making process. In about 1850, John Taylor of the Don Valley Paper Mill, Toronto, was the first to develop and later patent a Canadian process for making wood pulp. The use of wood as a cellulosic feedstock was the first major technological break-through in paper-making in 1,700 years. This advancement dramatically transformed the industry. Today in Canada, 144 large pulp and paper mills consumer vast quantities of wood, and produce both raw pulp and finished papers for the world market. THE PULP AND PAPER INDUSTRY AS A POLLUTION SOURCE To appreciate the sources of pollutants from this industry it is necessary to review the composition of the primary substrate, wood, and the chemical and mechanical processing that it undergoes to produce the quality pulps needed for paper-making. A. The Structure of Woody Materials(6) The three main component groups of wood are cellulose, hemicellulose, and lignin. Although the concentration of these three components can vary from species to species, the proportions are roughly 50% cellulose, 25% hemicellulose and 25% lignin. Cellulose is a very long linear molecule composed of repeating glucose units. Bundles of cellulose molecules from microfibrils, which build up to fibrils, and finally to cellulose fibres. Hydrogen bonding takes place between linear molecules to result in a strong microcrystalline structure. Cellulose, being nothing more than glucose in a tightly bound molecular structure, is quite susceptible to degradation by microorganisms. In spite of this, cellulose is generally not easily attacked by bacteria and moulds, because of the protective hemicellulose and lignin covering that enwraps the cellulose fibres. Hemicellulose is composed of a random arrangement of five-carbon sugars. These long-branching molecules surround the cellulose fibres, and intrude into pores in the cellulose. Hemicellulose forms chemical bonds with the next layer, lignin, and essentially acts as a chemical bonding agent between cellulose and lignin. Lignin is a complex phenylpropanoid polymer that surrounds and gives strength to the cellulose-hemicellulose framework. Lignin is composed of three phenol-based building blocks that polymerize in a completely random fashion. This random structure is very difficult to degrade. When slow degradation does take place, phenolic compounds, which are generally toxic, are released. As a result on a limited number of microorganisms derive benefit from the biological breakdown of lignin. The aromatic content of lignin, expressed as monomeric phenol, is approximately 51%. It is the phenolic compounds released from lignin during the chlorine bleaching of pulp that are responsible for a large percentage of the toxic compounds released in pulp mill effluents. In addition, wood contains 1.5 – 5% extractives, a general designation for wood components that may be extracted by organic solvents. These include resin acids, fats, waxes, terpenoid compounds, tannins, flavonoids, stilbenes, and troppolines. While extractives are generally present in mill wastes at much lower concentrations than phenolic materials, some of these compounds are relatively toxic, and can significantly reduce water quality and marine habitat in the lakes and rivers that receive mill effluents. The purpose of pulping is to free the cellulose fibres from the other wood components in as pure and undamaged a condition as possible. There are many pulping procedures; these evolve and change as economic and market circumstances dictate. The first widely used pulping method was the stone-groundwood process; this had the advantages of high yield and little waste, but resulted in damaged fibres that lacked strength. This process decreased in popularity when chemical pulping, which produced longer, stronger fibres, was developed.(7) During the early half of the twentieth century, sulphite pulping predominated in Canada, until it was replaced by Kraft pulping. Today Kraft mills account for a very large share of total pulp production. For example, in 1984 46.5% of all Canadian pulp was produced by the Kraft process.(8) Besides the production of high quality fibre, the other major advantage is the spent-liquor system, which recovers tall oil (fatty and resin acids), turpentine, bioenergy, and recycles inorganic chemicals.(9) The Kraft process is now being challenged by chemithermo-mechanical pulping (CTMP). In the CTMP process, chemistry, temperature, and mechanical parameters can be varied to optimize the release of cellulose from specific wood species. The economic advantages of CTMP are many: strength, brightness and versatility of the pulp produced; lower energy requirements; less need for processing; reduced water usage; the wide range of wood species that can be used; and, most important, the extremely high yield of pulp (90-92% of available cellulose fibre versus 40–42% for Kraft pulping). Unfortunately, the new developments that make the pulp and paper industry more competitive are not necessarily designed to reduce pollution. The basic CTMP process, unlike Kraft pulping, does not incorporate a recycling-recovery step. With CTMP, the reduced water flow, combined with higher fibre recovery that releases large amounts of lignin and extractives, greatly increases the potential for release of concentrated toxic effluents.(10) This problem is exacerbated if the organic waste stream receives chlorine-bleaching effluents. CTMP plants could have been devastating to the Canadian environment had they been built 20 years ago. Stringent pollution regulations are now in force, however, and, as new CTMP mills are built, they incorporate the necessary pollution control devices to ensure that mill effluents pass toxicity monitoring. In fact, the new goal of the pulp and paper industry is the development of "zero-emission" or "closed-loop" CTMP mills, where all wastes are recovered and process water is recycled. There are very few sulphite pulp mills still operating in Canada, and the newly built CTMP mills generally meet pollution effluent standards. This leaves Kraft pulping as the primary source of toxic pulp mill effluents. Figure 1 and the following brief overview of the operation of a conventional Kraft pulp mill and bleach plant describe pollution sources. Figure 1. General Flow Diagram for a Kraft Pulp Mill and Bleach Plant Source: Kringstad and Lindstrom (1984). Logs and debarked and then put through a chipper. The pulping process beings by treating wood chips at 160 – 180oC in a "white liquor" solution of sodium sulphide and sodium hydroxide. This treatment cleaves lignin ether bonds; and dissolves 90 to 95% of the lignin, essentially all hemicellulose, and wood extractives, and a small amount of cellulose-derived polysaccharides. Approximately 55% of the original wood is dissolved in what is now termed the "black liquor." By-products are recovered, and the liquor evaporated to high concentration and then burned for recovery of energy and inorganic chemicals.(11) The evaporated phase, which may contain inorganic sulphur in the form of sulphate, sulphite, or dithionite, is trapped in a condenser producing a concentrated sulphur waste water stream termed evaporator condensate. Highly volatile fugitive emissions from the condenser, and volatile compounds and gases from the burnt concentrated black liquor are released to the atmosphere. Air contaminants released from pulping include particulate matter, sulphur dioxide, and total reduced sulphur (TRS) compounds. The TRS compounds consist primarily of hydrogen sulphide, methyl mercaptan, dimethyl sulphide, and dimethyl disulphide. It is these compounds that contribute to the characteristic foul odors associated with pulp mills. The very low odour threshold of these pollutants means that disagreeable odours can be discerned by smell at concentrations that are seldom hazardous to human health. Accordingly, the main effect of pulp mill airborne emissions is the reduction of aesthetic air quality, although, the blackening by hydrogen sulphide of homes and buildings in proximity to pulp mills is not uncommon.(12) The pulping process is terminated when the pulp still contains 5 – 10% lignin, as further delignification would harm fibre quality. In the bleach plant the pulp is made into a 3% slurry and treated with a chlorine charge of 60 – 70 kg/t, at pH 1.5 – 2.0. This is followed by filtration and then an alkali treatment (35 – 40 kg/t), pH 11, at 55 – 70oC. Subsequent bleaching treatments may vary, but usually proceed through a series of hypochlorite, chlorine dioxide, alkali, and chlorine dioxide processing steps. After each treatment stage the pulp is filtered and the process liquids combined as bleachery effluent.(13) During bleach plant treatment, approximately 1 kg of extractives, 19 kg of polysaccharides, and 50 kg of lignin are dissolved from 1 tonne of softwood pulp. Chlorine can react with all of these organic wastes to produce structurally diverse organochlorine compounds. However, most reactions take place with the lignin fraction producing simple but toxic monoaromatic compounds such as chlorinated phenols, guaiacols, and catechols, as well as high molecular weight chlorolignins. The latter compounds are thought to be nontoxic, as their large size precludes their penetration or transport across cell membranes. In spite of this, they are not environmentally benign, as they carry chromophoric structures that discolour receiving waters. Also, there is the potential for the release of toxic compounds as the chlorolignins slowly degrade.(14)(15)(16) Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzo-p-furans (PCDFs) have been identified in emissions from the pulp industry. Their concentration in stack gases from the burning of condensed black liquor is very low. In Sweden it has been estimated that the annual production of 4 million tonnes of pulp results in approximately 2 grams of PCDD and PCDF congeners emitted into the air.(17) PCDDs and PCDFs also are produced during pulp bleaching, where they are formed from chlorinated phenols, and particularly from chlorinated 2-phenoxyphenols. Bleachery effluents account for the release of 5 – 15 grams total PCDD and PCDF congeners per year in Sweden. Accordingly, the Swedish pulp industry is responsible for only a very small amount (1.7%) of the approximately 1 kg of PCDDs and PCDFs released from all sources in that country. This has been verified by comparison of isomer patterns. The pulp industry produces primarily 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin and 2, 3, 7, 8-tetrachloro-dibenzo-p-furan, while the isomer profiles from incinerator operations are distinctly different.(18) Similarly, in the United States, it was estimated that in 1990 the pulp and paper industry would be contributing only 1.5% of the approximately 40 kg of total PCDDs and PCDFs released to the environment annually.(19) Although total environmental contribution is quite low, the pulp and paper industry has spent large sums of money on research and development to eliminate or at least greatly reduce these compounds in effluents and paper products. The industry is threatened by publicity and public fear of dioxins, which peaked with the discovery of dioxin in milk cartons. It has been possible to reduce the dioxin level in milk carton cardboard to 2 parts per trillion, a level indistinguishable from typical background levels in milk.(20) IDENTIFICATION OF COMPOUNDS HAZARDOUS TO HUMAN HEALTH During the past century many chlorinated organic pesticides, solvents, refrigerants, etc., that have been chemically synthesized, have been found to be highly toxic, and/or extremely difficult to degrade and remove from the environment. These man-made chemicals are called xenobiotics. They are not natural chemicals, in that they have never been produced in nature by animal or plant metabolism. As such, they have no natural enemies; that is, microorganisms have not been in contact with these chemicals long enough to evolve the enzyme systems necessary for their rapid degradation. Similarly, many of the chlorinated organic compounds randomly synthesized during pulp bleaching are toxic xenobiotics that will persist for long periods in the environment. The situation can often be made worse if pulp mill effluents are released to oxygen-limited or depleted (anaerobic) waters. Certain species of anaerobic bacteria can methylate chlorinated organic compounds, thereby making the compounds less toxic to the bacteria. Unfortunately, this mechanism generally increases both the toxicity and lipophilicity of the compound to higher animals. Lipophilic compounds are preferentially soluble in fats. When a methylated-chlorinated compound is consumed by fish or fowl, rather than being completely eliminated in the urine it may become sequestered in body fat in a phenomenon termed "bioaccumulation." The toxic compound becomes more and more concentrated as it is carried up the food chain.(21) Wood is approximately 50% cellulose. this means, even after taking into consideration by-product recovery, the volume and variety of organic and chlorinated organic wastes generated by the pulp and paper industry is immense. The magnitude of this waste problem necessitates the identification of those chemicals that pose a significant threat to human health. the identification of toxic, mutagenic, and potentially carcinogenic compounds and the waste streams that produce them is essential in order to mitigate them at source and to develop technologies for their safe degradation. Although a large variety of chlorinated compounds are present in pulp mill effluents, they are seldom at concentrations that would cause acute toxicity. For example, the minimum lethal oral doses (for human beings) of the common bleach effluent compounds trichloromethane and pentachlorophenol are 14.8 grams and 1.2 grams respectively for a 70 kg adult.(22)(23) Accordingly, the danger to human health from organochlorines emanating from pulp and paper mills is primarily through long-term exposure via drinking water, and bioaccumulation through the consumption of contaminated fish. The toxic compounds most often identified in aquatic life below pulp and paper mill effluent discharge points are tri-, and tetrachloroguaiacols, tetrachlorocatechols and di-, tri-, tetra-, and pentachlorophenols.(24)(25) Also of concern is the lignin breakdown product acetovanillone, which has been found to undergo chemical degradation and chlorination to produce toxic chlorinated acetones. The most toxic of these is 1, 1, 3-trichloroacetone which has been found in bleachery effluents at concentrations up to 2.4 mg/litre.(26) Considerable attention has also been directed toward identification of the mutagenic (and thereby potentially carcinogenic) compounds in pulp mill effluents. Strategies for the evaluation of pulp-mill effluents for mutageni potential have generally followed three approaches: assessment of whole effluent streams, of fractionated effluent streams, and of pure compounds known to be effluent components. In the first approach, authentic waste solutions are tested, in which chemical and physical interactions and possible synergistic effects are maintained. The many studies performed on whole pulp mill waste streams are all in agreement that the first-chlorination-stage effluent shows high mutagenic activity, while other effluents and process streams show no or reduced mutagenicity.(27) Examination of fractionated effluent streams, and the individual testing of approximately 300 known constituent chemicals of pulp mill effluents, showed that 39 compounds exhibited weak to very strong mutagenic activity. Two resin acids (neoabietic and 7-oxodehydroabietic acid), two chlorinated spirodiones, tri-, tetra-, penta-, and hexachloroacetone, and a number of chlorinated alipathic hydrocarbons were identified as mutagenic agents.(28)(29) Chloroacetones, although demonstrating substantial mutagenicity, do not pose a serious human health risk as these compounds are quite unstable and tend to degrade rapidly in receiving waters.(30) On the other hand, chlorinated aliphatic hydrocarbons, such as trichloro- and tetrachloroethylene, and their breakdown products are known mammalian carcinogens.(31) The observation that two resin acids exhibited mutagenic activity is of concern, as these acids degrade very slowly in anaerobic environments. Resin acids have also been found to bioaccumulate in fish at sublethal concentrations. The combination of persistence, bioaccumulation, and mutagenic activity makes these compounds a potential health risk.(32) The most potent bacterial mutagen in pulp mill effluents was identified as 3-chloro-4-(dichloromethyl)- 5-hydroxy-2(5H)-furanone (MX). This compound was found to be responsible for 30 to 50% of the mutagenicity of chlorination-stage effluents. In the Ames test, MX demonstrated a mutagenic potential nearly two-fold greater than the extremely potent mould metabolite aflatoxin B1.(33) This is therefore a compound of concern. Research has shown that MX stability decreases with increasing pH in the range of pH 2 – 6. Most waste water treatment facilities operate at a pH of 6 – 7. Accordingly, MX should be deactivated and destroyed at pulp mills where the bleach plant effluent goes directly for conventional waste water treatment. However MX could pose a serious risk if mill effluents were discharged directly to receiving waters. This is particularly so in Canada and Northern Europe where lake and river waters may be slightly acidic due to poor buffering capacity, high humic acid content, and acid rain. Dioxins are routinely described in the news media as extremely dangerous, toxic, cancer-causing agents. In spite of the extreme public fear of dioxins, it has not been established that the polychlorinated dibenzo-p-dioxins and polychlorinated dibenzo-p-furans pose a serious threat to human health. There is some indication, however, that dioxins increase the risk of soft-tissue sarcomas in heavily exposed industrial workers; and, acute dioxin exposure is known to have caused nausea and a relatively long-lasting (2-3 years) skin condition called chloracne.(34)(35) There have been no human deaths from acute toxicity of dioxins, even though over 200 people in Nitro, West Virginia, in 1949, and 37,000 people in Seveso, Italy, in 1976, were possibly exposed to dioxin-laden dust. In these incidents many contaminated animals died; however, human suffering was limited to nausea and cases of severe chloracne. Follow-up studies have not shown an increased incidence of cancer or birth defects. Although dioxin and furan cogeners do not appear to be highly toxic to human beings, they are extremely toxic to certain species of animals. For example, in the guinea pig the LD50 dosage for dioxin is 0.6 micrograms/kg; while the LD50 concentration for hamsters is about 5000 times greater.(36) Many countries are now legislating discharge limits for pulp and paper mill effluents. For 1991 the Swedish permissible discharge limit was set at 1.2 to 1.3 kg total organic chlorine per tonne of pulp.(37) This means that for each tonne of pulp produced, only 1.3 kg of organic material that is chemically reacted (combined) with chlorine can be discharged to the aqueous environment. Three Canadian provinces have initiated discharge regulations using the more stringent generic classification of Absorbable Organic Halogen (AOX).(38) This is more stringent, because it includes all halogen compounds (chlorine, fluorine, bromine, etc.) that might be chemically bonded or absorbed to organic matter issuing from a pulp mill. The Ontario discharge limit was set at 2.5 kg AOX/tonne pulp, with a further reduction to 1.5 kg AOX/tonne pulp by 1993. A similar preliminary limit was set in British Columbia, with mandatory secondary treatment in 1991, and reduction to 1.5 kg AOX/tonne pulp by the end of 1994. In Quebec AOX levels of 1.5 kg/tonne pulp and zero detectable levels for dioxins and furans are to be met by 31 December 1993(39)(40) For comparison, the average AOX discharge in untreated effluent from a Kraft mill is 8.0 kg per tonne of softwood pulp.(41) In December 1991, the Canadian government announced new federal regulations to control pulp mill pollution. As of 1 January 1994, any measurable level of dioxins or furans in pulp mill effluent will constitute a violation of the regulations. In addition, wood chips that might be contaminated with preservatives and certain defoaming agents can no longer be used in the pulping process as they might contribute to the release of toxic pollutants.(42) While these regulations are national in scope, each province has the right to impose even more stringent regulations within its own jurisdiction. Of the many pollutants in pulp and paper mill effluents, only dioxins and furans have been evaluated by Health and Welfare Canada as "priority substances." Dioxins and furans were declared to be toxic and to pose a possible risk to human health. For this reason, the federal government legislated specifically on dioxins and furans and did not include other known pollutants. This was not a "half-hearted" attempt at regulation, as some environmental groups have claimed; rather, it is expected that the process changes and the product substitution necessary to prevent the formation of dioxins and furans will have the effect of reducing the overall release of chlorinated organic compounds to less than 2 kg AOX/t. Meeting these discharge limits will not be easy. In some cases it will mean the earlier closure of out-dated plants as the pulp and paper industry phases out old sulphite and Kraft mills and replaces them with new, more competitive, chemithermomechanical pulping plants. Compliance ill definitely mean the adoption of alternative methods to reduce chlorine bleaching and the establishment of high-efficiency treatment facilities. Attainment of pollution abatement goals will also be very expensive. For example, Canadian Pacific Forest Products Ltd. spent $60-million on state-of-the-art treatment facilities for a pulp mill in Dryden, Ontario. This mill now meets Ontario AOX discharge regulations.(43) POLLUTION ABATEMENT BY THE PULP AND PAPER INDUSTRY Research and the development of new processes and techniques to curtail the release of toxic effluents from pulp and paper mills are following two approaches. The first strategy is the development of new pulping processes with emphasis on improved delignification, and the replacement or partial replacement of chlorine for bleaching. The second approach is the development of new biological treatment processes, particularly hybrid or dual systems that capitalize on the advantages afforded by both anaerobic and aerobic digestion. A new process called oxygen delignification has been found to be quite effective in removing the lignin and hemicellulose fraction from cellulose, and leaving the fibres relatively undamaged. The major environmental advantage is greater lignin removal, so that less lignin enters the bleach plant. No toxic chlorinated organic compounds would be released in mill effluents if chlorine could be completely eliminated from the pulp-bleaching process. Partial replacement of chlorine by chlorine dioxide has been found to reduce greatly the emission of organochlorines, while complete substitution with hydrogen peroxide eliminates these toxic compounds. The use of hydrogen peroxide gives the additional benefit of assisting in the breakdown of other organic contaminants in the effluent through chemical oxidation reactions. Pulp bleached by hydrogen peroxide is not, however, as white as chlorine-bleached pulp. B. End-of-Pipe Biological Treatment Processes At present, most pulp and paper mills use aerated lagoons to treat their wastes prior to discharging them into receiving waters. This is done to ensure that the effluents meet Biological Oxygen Demand (BOD) discharge limits and pass fish toxicity tests. More sophisticated and expensive forms of waste water treatment will be required, however, if the pulp and paper industry is to meet the new, more stringent, discharge limits set for absorbable organic halogens. It is essential that these new treatment technologies be designed to degrade those chemicals that pose the greatest threat to human health. the chemicals of concern are those that are toxic and/or mutagenic, have a tendency to bioaccumulate, and are difficult to degrade. Using these criteria, the degradation of all mutagenic compounds, resin acids, and chlorinated phenols, guaiacols, catechols, and chlorinated aliphatic hydrocarbons should be the focus of new treatment processes. As previously stated, highly chlorinated compounds are quite stable and difficult to degrade. It is possible, however, for bacteria in the anaerobic environment (no oxygen) to conduct a transformation reaction whereby chlorine ions are replaced by hydrogen ions. The more chlorine ions that are removed, the more reactive the compound becomes, and the more susceptible to aerobic (oxygen present) microbial degradation in a conventional activated sewage sludge digester.(44)(45) Accordingly, the establishment of sequential anaerobic-aerobic waste-water treatment facilities at Kraft pulp and paper mills will greatly help these plants to reduce their toxic emissions. Sequential anaerobic-aerobic treatment also effectively degrades mutagenic compounds and chlorinated aliphatic compounds, which are known mammalian carcinogens. However, resin acids cannot be degraded in an anaerobic environment. In fact, resin acids are highly toxic to anaerobic bacteria, and can cause an anaerobic waste-water system to fail.(46) Therefore, the sequential system is not recommended for CTMP wastes that often contain high concentrations of resin acids. However, these acids can be degraded by aerobic digestion. In Sweden a three-stage process of aerobic-anaerobic-aerobic has been investigated and found to degrade both resin acids and organochlorines successfully.(47) C. Pollution Abatement – A Case Study Over a 15-year period, alterations in pulp processing and waste treatment at the E.B. Eddy Forest Products Ltd. Kraft mill in Espanola, Ontario, have enabled this mill not only to meet but to exceed the Ontario 1993 discharge limit of 1.5 kg AOX/t pulp. In 1970, this mill discharged effluent directly into the Spanish River, polluting the river for 32 km, and upsetting the benthic population to the mouth of the river, a distance of 52 km. In 1977, North America’s first oxygen delignification process was started as an extension of the pulp-cooking process. This resulted in 50% lower lignin in put to the bleach plant, increased pulp brightness and strength, and a 23% reduction in bleaching cost. Biological treatment was implemented in 1983. This consisted of a 12-hour settling basin (primary treatment), a 6-day aerated lagoon (secondary treatment), and a 12-hour quiescent zone to settle biosolids. Strict controls were put in place. Evaporator condensate was steam-stripped and burnt. Black liquor soap scum was skimmed and processed into tall oil. As well, in-plant spill collection and a sewer monitoring system were established. These controls all served to protect the microbial population of the lagoon from shock loads of effluent. Finally, in 1988 chlorine dioxide substitution for chlorine bleaching was instituted. At 52% substitution, dioxins and furans could not be detected in pulp or in bleachery effluents, and AOX effluent emissions were reduced to below 1 kg/t pulp. The environmental improvements at this mill have been so pronounced that the Spanish River is no longer polluted and supports pickerel fishing. In addition, the quiescent zone and outfall pond support cattails, ducks and beavers.(48) Many pulp and paper mills use chlorine as a bleaching agent to produce high-quality white pulp. The high organic content of mill waste, coupled with the presence of chlorine, results in the production of many highly toxic chlorinated organic compounds. Of prime concern are chlorinated phenols, guaiacols, catechols, furans, dioxins, aliphatic hydrocarbons and the highly mutagenic agent MX. In addition, two natural wood resin acids, neoabietic and 7-oxodehydroabietic acids, exhibit mutagenic activity. These compounds pose a human health risk through long-term exposure via drinking water, and through bioaccumulation along the food chain. The pulp and paper industry is starting to implement new technologies that greatly lower the concentration of toxic substances in mill effluents. In-plant reduction of toxic emissions is being effected through the installation of new oxygen delignification processes, and replacement or partial replacement of chlorine by hydrogen peroxide or chlorine dioxide for pulp bleaching. End-of-pipe clean-up of mill effluents can be accomplished by new biological treatment processes, such as sequential anaerobic-aerobic digestion. Stringent regulation f of pulp and paper mill discharge by provincial and federal authorities will necessitate large capital expenditures by the industry. This may mean the early closure of old mills, and economic hardship for many communities. At the same time, however, these regulations will ensure improved environmental quality and a reduction of the risk to human health that is posed by this vital industry. Axegard, P. "Improvement of Bleach Plant Effluent by Cutting Back on C12." Pulp and Paper Canada, Vol. 90, 1989, p. 78-81. Basta, J. et al. "Low AOX, Possibilities and Consequences." 1989 Pulping Conference, Tappi Press, Atlanta, 1989, p. 427-436. Carruthers, George. Paper in the Making. Garden City Press Coop., Toronto, 1947, 712 p. Cheremisinoff, P.N. "High Hazard Pollutants: Asbestos, PCBs, Dioxins, Biomedical Wastes." Pollution Engineering, Vol. 21, 1989, p. 58-65. Crittenden, G. "Operation Zero." Hazardous Materials Management, Vol. 2, 1990, p. 8-11. Douglas, G.R. et al. "Mutagenic Activity in Pulp Mill Effluents." Water Chlorination: Environmental Impact and Health Effects. R.L. Jolley et al. (eds.). Vol. 3, Ann Arbor Science, Ann Arbor, 1980, p. 865-880. Douglas, G.R. et al. "Mutagenicity of Pulp and Paper Mill Effluent: a Comprehensive Study of Complex Mixtures." Short-Term Bioassays in the Analysis of Complex Environmental Mixtures III. M.D. Waters, et al. (eds.). Plenum Press, New York, 1983, p. 431-459. Dreisbach, R.H. Handbook of Poisonings: Prevention, Diagnosis, and Treatment. 10th ed. Lange Medical Publ., Los Altos, California, 1980, 364 p. Dubelstein, P. and N.C.C. Gray, "The Effects of Secondary Treatment on AOX Levels in Kraft Mill Effluents." 76th Annual Symposium of the Canadian Pulp and Paper Association, Montreal, 1990, p. 317-324. Environment Canada. Emissions and Trends of Common Air Contaminants in Canada: 1970-1986. Report EPS 7/AP/17. Environment Canada, Ottawa, 1986, 104 p. Environment Canada. New Federal Regulations to Control Pulp Mill Pollution. News Release PR-HQ-091-45. Environment Canada, Ottawa, 1991. Haggblom, M. "Mechanisms of Bacterial Degradation and Transformation of Chlorinated Monoaromatic Compounds." Journal of Basic Microbiology, Vol. 30, 1990, p. 115-141. Hall, Eric R. et al., "Organochlorine Discharges in Wastewaters from Kraft Mill Bleach Plants." Environmental Conference of the Canadian Pulp and Paper Association, Vancouver, 1988, p. 53-62. Kierkegaard, A. and L. Renberg. "Chemical Characterization of Organochlorine Compounds, Originating from Pulp Mill Effluents in Fish." Water Science and Technology, Vol. 20, 1988, p. 165-170. Kringstad, K.P. and K. Lindstrom. "Spent Liquors from Pulp Bleaching." Environmental Science and Technology, Vol. 18, 1984, p. 236A-247A. McConnell, E.E. et al. "The Comparative Toxicity of Chlorinated Duibenzo-p-dioxin Isomers to Mice and Guinea Pigs." Toxicology and Applied Pharmacology, Vol. 37, 1976, p. 146-153. McKague, A.B. et al. "Chloroacetones in Pulp Mill Chlorination Stage Effluents." Environmental Toxicology and Chemistry, Vol. 9, 1990, p. 1301-1303. McKague, A.B. et al. "Chloroacetones: Mutagenic Constituents of Bleached Kraft Chlorination Effluent." Mutation Research, Vol. 91, 1981, p. 301-306. Meier, J.R. et al. "Studies on the Potent Bacterial Mutagen, 3-chloro-4-(dichloromethyl)-5-hydroxy- 2(5H)-furanone: Aqueous Stability, XAD Recovery and Analytical Determination in Drinking Water and in Chlorinated Humic Acid Solutions." Mutation Research, Vol. 189, 1987. p. 363-373. Mikesell, M.D. and S.A. Boyd. "Complete Reductive Dechlorination and Mineralization of Pentachlorophenol by Anaerobic Microorganisms." Applied and Environmental Microbiology, Vol. 52, 1986, p. 861-865. Miller, R.E. and F.P. Guengerich. "Metabolism of Trichloroethylene in Isolated Hepatocytes, Microsomes, and Reconstituted Enzyme Systems Containing Cytochrome P450." Cancer Research, Vol. 43, 1983, p. 1145-1152. Munro, F.C. "Environmentally Influenced Evolution of E.B. Eddy Espanola’s Bleaching Sequences." Bleach Plant Operations, Tappi Press, Atlanta, 1990, p. 13-24. Nazar, M.A. and W.H. Rapson. "pH Stability of Some Mutagens Produced by Aqueous Chlorination of Organic Compounds." Environmental Mutagenesis, Vol. 4, 1982, p. 435-444. Neilson, A.H. et al. "The Environmental Fate of Chlorophenolic Constituents of Bleachery Effluents." Tappi Journal, Vol. 73, 1990, p. 239-247. Nestmann, E.R. et al. "Mutagenicity of Resin Acids Identified in pulp and Paper Mill Effluents Using the Salmonella/Mammalian Microsome Assay." Environmental Mutagenesis, Vol. 1, 1979, p. 361-369. Paasivirta, J. et al. "Transportation and Enrichment of Chlorinated Phenolic Compounds in Different Aquatic Food Chains." Chemosphere, Vol. 9, 1980, p. 441-456. Paasivirta, J. et al. "Polychlorinated Phenols, Guaiacols, and Catechols in Environment." Chemosphere, Vol. 14, 1985, p. 469-491. Schroeder, H.G. "Acute and Delayed Chloroform Poising: A Case Study." British Journal of Anaesthesia, Vol. 37, 1965, p. 972-975. Sierra-Alvarez, R. and G. Lettinga. "The Methanogenic Toxicity of Wood Resin Constituents." Biological Wastes, Vol. 33, 1990, p. 211-226. Sinclair, William F. Controlling Pollution from Canadian pulp and Paper Manufacturers: A Federal Perspective. Environment Canada, Ottawa, 1990, 360 p. Springer, A.M. Industrial Environmental Control: Pulp and Paper Industry. John Wiley and Sons, N.Y., 1986, p. 3. Stillman, R. "Dioxin." Hazardous Materials Management, Vol. 2, 1990, p. 38-42. Swanson, S.E. et al. "Emissions of PCDDs and PCDFs from the Pulp Industry." Chemosphere, Vol. 17, 1988, p. 681-691. Tana, J.J. "Sublethal Effects of Chlorinated Phenols and Resin Acids on Rainbow Trout Dslmo gairdneri)." Water Science Technology, Vol. 20, 1988, p. 77-85. Welander, T. "An Anaerobic Process for Treatment of CTMP Effluent." Water Science Technology, Vol. 20, 1988, p. 143-147. (1) A.M. Springer, Industrial Environmental Control: Pulp and Paper Industry, John Wiley and Sons, N.Y., 1986, p. 3. (2) William F. Sinclair, Controlling Pollution from Canadian Pulp and Paper Manufacturers: A Federal Perspective, Environment Canada, Ottawa, 1990, 360 p. (3) Environment Canada, Emissions and Trends of Common Air Contaminants in Canada: 1970-1980, Report EPS 7/AP/17, Environment Canada, Ottawa, 1986, 104 p. (4) George Carruthers, Paper in the Making, Garden City Press Coop., Toronto, 1947, 712 p. (5) Ibid. (6) K.P. Kringstad and K. Lindstrom, "Spent Liquors from Pulp Bleaching," Environmental Science and Technology, Vol. 18, 1984, p. 236A-247A. (7) Sinclair (1990). (8) Ibid. (9) Kringstad and Lindstrom (1984), p. 236A-247A. (10) Sinclair (1990). (11) Kringstad and Lindstrom (1984), p. 236A-247A. (12) Sinclair (1990). (13) Kringstad and Lindstrom (1984), p. 236A-247A. (14) Ibid. (15) A.H. Neilson et al., "The Environmental Fate of Chlorophenolic Constituents of Bleachery Effluents," Tappi Journal, Vol. 73, 1990, p. 239-247. (16) J. Paasivirta et al., "Polychlorinated Phenols, Guaiacols, and Catechols in Environment," Chemosphere, Vol. 14, 1985, p. 469-491. (17) S.E. Swanson et al., "Emissions of PCCDs and PCDFs from the Pulp Industry," Chemosphere, Vol. 17, 1988, p. 681-691. (18) Ibid. (19) R. Stillman, "Dioxin," Hazardous Materials Management, Vol. 2, 1990, p. 38-42. (20) Ibid. (21) A. Kierkegaard and L. Renberg, "Chemical Characterization of Organochlorine Compounds, Originating from Pulp Mill Effluents in Fish," Water Science and Technology, Vol. 20, 1988, p. 165-170. (22) H.G. Schroeder, "Acute and Delayed Chloroform Poisoning: A Case Study," British Journal of Anaesthesia, Vol. 37, 1965, p. 972-975. (23) R.H. Dreisbach, Handbook of Poisonings: Prevention, Diagnosis, and treatment, 10th ed., Lange Medical Publ., Los Altos, California, 1980, 364 p. (24) J. Paasivirta et al., "Transportation and Enrichment of Chlorinated Phenolic Compounds in Different Aquatic Food Chains," Chemosphere, Vol. 9, 1980, p. 441-456. (25) J.J. Tana, "Sublethal Effects of Chlorinated Phenols and Resin Acids on Rainbow Trout (Salmo gairdneri)," Water Science Technology, Vol. 20, 1988, p. 77-85. (26) A.B. McKague et al., "Chloroacetones in Pulp Mill Chlorination-stage Effluents," Environmental Toxicology and Chemistry, Vol. 9, 1990, p. 1301-1303. (27) G.R. Douglas et al., "Mutagenic Activity in Pulp Mill Effluents," Water Chlorination: Environmental Impact and Health Effects, R.L. Jolley et al. (eds.), Vol. 3, Ann Arbor Science, Ann Arbor, 1980, p. 865-880. (28) G.R. Douglas et al., "Mutagenicity of Pulp and Paper Mill Effluent: a Comprehensive Study of Complex Mixtures," Short-Term Bioassays in the Analysis of Complex Environmental Mixtures III, M.D. Waters et al. (eds.), Plenum Press, New York, 1983, p. 431-459. (29) A.B. McKague et al., "Chloroacetones: Mutagenic Constituents of Bleached Kraft Chlorination Effluent," Mutation Research, Vol. 91, 1981, p. 301-306. (30) M.A. Nazar and W.H. Rapson, "pH Stability of Some Mutagens Produced by Aqueous Chlorination of Organic Compounds," Environmental Mutagenesis, Vol. 4, 1982, p. 435-444. (31) R.E. Miller and F.P. Guengerich, "Metabolism of Trichloroethylene in Isolated Hepatocytes, Microsomes, and Reconstituted Enzyme Systems Containing Cytochrome P450," Cancer Research, Vol. 43, 1983, p. 1145-1152. (32) E.R. Nestmann et al., "Mutagenicity of Resin Acids Identified in Pulp and Paper Mill Effluents Using the Salmonella/Mammalian-Microsome Assay," Environmental Mutagenesis, Vol. 1, 1979, p. 361-369. (33) J.R. Meier et al., "Studies on the Potent Bacterial Mutagen, 3-chloro-4-(dichloromethyl)-5-hydroxy- 2(5H)-furanone: Aqueous Stability, XAD Recovery and Analytical Determination in Drinking Water and in Chlorinated Humic Acid Solution," Mutation Research, Vol. 189, 1987, p. 363-373. (34) R. Stillman (1990), p. 38-42. (35) P.N. Cheremisinoff, "High Hazard Pollutants: Asbestos, PCBs, Dioxins, Biomedical Wastes," Pollution Engineering, Vol. 21, 1989, p. 58-65. (36) E.E. McConnell et al., "The Comparative Toxicity of Chlorinated Dibenzo-p-dioxin Isomers to Mice and Guinea Pigs," Toxicology and Applied Pharmacology, Vol. 37, 1976, p. 146-153. (37) J. Basta et al., "Low AOX, Possibilities and Consequences," 1989 Pulping Conference, Tappi Press, Atlanti, 1989, p. 427-436. (38) P. Axegard, "Improvement of Bleach Plant Effluent by Cutting Back on C12," Pulp and Paper Canada, Vol. 90, 1989, p. 78-81. (39) P. Dubelsten and N.C.C. Gray, "The Effects of Secondary Treatment on AOX Levels in Kraft Mill Effluents," 76th Annual Symposium of the Canadian Pulp and Paper Association, Montreal, 1990, p. 317-324. (40) G. Crittenden, "Operation Zero," Hazardous Materials Management, Vol. 2, 1990, p. 8-11. (41) Eric R. Hall et al., "Organochlorine Discharges in Wastewaters from Kraft Mill Bleach Plants," Environmental Conference of the Canadian Pulp and Paper Association, Vancouver, 1988, p. 53-62. (42) Environment Canada, New Federal Regulations to Control Pulp Mill Pollution, News Release Pr-HQ-091-45, Ottawa, Environment Canada, 1991. (43) Crittenden (1990), p. 8-11. (44) M.D. Mikesell, and S.A. Boyd, "Complete Reductive Dechlorination and Mineralization of Pentachlorophenol by Anaerobic Microorganism," Applied and Environmental Microbiology, Vol. 52, 1986, p. 861-865. (45) M. Haggblom, "Mechanisms of Bacterial Degradation and Transformation of Chlorinated Monoaromatic Compounds," Journal of Basic Microbiology, Vol. 30, 1990, p. 115-141. (46) R. Sierra-Alvarez and G. Lettinga, "The Methanogenic Toxicity of Wood Resin Constituents," Biological Wastes, Vol. 33, 1990, p. 211-226. (47) T. Welander, "An Anaerobic Process for Treatment of CTMP Effluent," Water Science Technology, Vol. 20, 1988, p. 143-147. (48) F.C. Munro, "Environmentally Influenced Evolution of E.B. Eddy Espanola’s Bleaching Sequences," Bleach Plant Operations, Tappi Press, Atlanta, 1990, p. 13-24. |