|
BP-293E
OZONE: THE EARTH'S SUNSCREEN
Prepared by: TABLE
OF CONTENTS B. Technological Alternatives to the Agents ACTION TAKEN TO REDUCE THE DESTRUCTIVE AGENTS B. Canadian Federal-Provincial Action
OZONE: THE EARTH'S SUNSCREEN Life on earth is a delicate balance of many different elements. The conditions necessary for the diversity and abundance of life are fairly restricted and any major change can have unpredictable results. The raw energy of the sun maintains all forms of life on the planet. One of the main components of the sun’s energy transmitted to the earth is ultraviolet radiation. This radiation can be very useful in moderation; in excess, it can cause serious problems to mankind and to the biosphere in general. One of the main mechanisms that moderate this radiation is the ozone in the stratosphere. It could be said that the ozone layer acts as the earth’s sunscreen. Since the 1970s, we have known that various man-made products are destroying this life-preserving filter. Much has been done to attempt to improve the situation, which has become increasingly alarming. The recent preliminary finding of the National Aeronautics and Space Agency (NASA) suggests that the situation is worse than had been anticipated and that Canada can expect increased frequencies of "holes" in the ozone layer in the future. This paper will discuss the various aspects of the ozone situation, from technical explanations to the latest efforts to remedy the problem. The atmosphere is the earth’s protective blanket that enables life to exist. Life on earth evolved slowly so that each portion of the ecosystem is adapted to make best use of the available conditions. Over geological time periods, the earth’s atmosphere has changed. Human activity has caused many of the changes in the atmosphere and has itself slowly had to adapt to them. Mankind over the last century has been changing the composition of the earth’s atmosphere. Some changes, such as increasing the amount of pollution in the air we breathe and modifying the energy absorption characteristics of the atmosphere, will have a direct impact on mankind. The direct fouling of breathable air is very noticeable; however, modifications to the absorption and reflective qualities of the atmosphere are more long-term and more difficult to observe directly, particularly given the large number of interacting elements. Some of these changes include the reduced absorption of harmful ultraviolet radiation, which increases the reflection of energy back into space, and in turn the reflection of the reabsorbed heat energy, so as to trap the energy light in much the same way as a greenhouse does. One characteristic of the atmosphere changed by mankind is the quantity of ozone available to protect life on earth. To fully appreciate the importance of this, we need a basic understanding of the processes surrounding ozone change. Ozone (O3) is a pungent-smelling, slightly bluish gas, which is a close chemical cousin to molecular oxygen (O2). It can be found from ground level up to about 60 km; the stratosphere contains approximately 90% of all ozone. The stratospheric ozone has a peak concentration at 25 km and forms a layer about 20 km thick which is located between 15 km and 35 km above the earth’s surface. The ozone is so thin that if it were compressed to ground-level pressure, it would form a layer only 3 mm thick.(1) Ozone, particularly in the stratosphere, is the main filter that reduces the quantity of ultraviolet radiation reaching the earth’s surface. The net value of other filters for this radiation, which include some forms of atmospheric pollution and clouds, is not well documented. Ozone is formed in both the troposphere and the stratosphere. In the stratosphere, the ozone concentrations are more stable and the conditions are better known. Ozone (O3) is constantly produced when high energy radiation, such as ultraviolet radiation, causes oxygen molecules (O2) to split into two oxygen atoms and then recombine with another oxygen molecule. The formation and destruction of ozone develops new equilibrium points, depending on various conditions. Some processes are thought to increase the level of ozone. Increased concentrations of carbon dioxide (CO2) and methane (CH4), both "greenhouse gases," are predicted to increase the column content of ozone.(2) The level of solar radiation emitted by the sun can also directly affect ozone production. The production of ozone in the troposphere is much more dynamic and unpredictable. Although the general principles are similar to those governing the production of ozone in the stratosphere, the much more variable conditions in the troposphere reduce the stability of the concentrations. The process for the destruction of stratospheric ozone is very complex and involves numerous factors. It is well recognized that one of the primary agents responsible is chlorine. The process has recently come to be viewed as a "heterogenous" reaction(3) in which ice crystals or volcanic ash are used as platforms providing a surface on which the chemical reactions that suppress nitrogen oxides take place.(4) Additional strong influences on the process are the air circulation and weather patterns, particularly in the case of the ozone "holes" observed so far. Very cold weather can accelerate the formation of nitric acid, which plays a major role in the destruction of ozone. The various factors involved in the process can arise in different ways. The chlorine in the stratosphere can originate from numerous sources, both man-made and natural. The major man-made contributors of chlorine are chlorofluorocarbons (CFCs) and other similar material which slowly rise, over a period of five years, into the stratosphere, where they decompose. CFCs will remain in the atmosphere for more than 100 years. The surface material needed for the destructive process arises from man-made pollution, ice crystals and major volcanic activity. Although the destructive process involves many variables, a simplified view of what happens with CFCs will highlight its magnitude. CFCs break down in the stratosphere, the ultraviolet radiation "dissolves" the "bonds" that hold the chlorine and fluorine and carbon atoms together and liberates chlorine. Two distinct processes take place. In one of these, the chlorine atom (C1) reacts with ozone (O3) to form chlorine monoxide (C1O) and molecular oxygen (O2). In the other, chlorine oxide (C1O) combines with atomic oxygen (O) to form atomic chlorine (C1) and molecular oxygen (O2). Typically, one chlorine atom, acting as a catalyst, can destroy up to 100,000 ozone molecules. Any substantial reduction in the stratospheric ozone layer would increase the quantity of ultraviolet energy reaching the earth. The sun emits a wide spectrum of energy, whose amount varies over time and most forms of which are used to provide heat and light to the earth. Some is in the form of ultraviolet energy, which has a wavelength shorter than violet light. Unfortunately, the portion of the ultraviolet light with wavelengths from 280 to 320 nanometres, known as ultraviolet B (UV-B), is dangerous in excess. The harmful effects of an overabundance of UV-B fall on life directly, as well as indirectly by damaging the biosphere and modifying the atmosphere. The extent of the harm and the range of life forms affected are under investigation. Although some research is showing that limited adaptation is possible, most of the effects remain very negative. Most of the latest information on the impact of increased UV-B is from a recent United Nations Report, Environmental Effects of Ozone Depletion: 1991 Update,(5) which was released to the public only in February 1992. A large portion of the detailed material on the adverse effects of UV-B is extracted from this report. Human health, human cultivated food production and man-made materials are harmed by higher levels of UV-B. Most of the effects of excessive UV-B due to a reduction in the ozone layer have some degree of proportionality. The large number of complex interactions that occur can make it difficult to interpret the results. Several direct consequences of increased levels of UV-B on humans have been confirmed:
Other medical effects are still the subject of debate.
This widely publicized prediction can be questioned in view of other studies. A review on "Cutaneous Melanoma," published in 1991 by an epidemiologist from Boston University cited numerous studies indicating that skin cancer appeared relatively rarely in people with outdoor occupations.(8) The review also cited studies supporting the hypothesis that the risk of melanoma depends particularly on intermittent exposure to the sun, especially in early life.(9) Some crops show decreased production as a result of more elevated amounts of UV-B. Experiments have shown that a 1% reduction in the ozone layer results in a 1% reduction in the yield of soybeans.(10) Many crops are unaffected or show a high degree of tolerance, however. Some research indicates that the growth and photosynthesis of certain plants (e.g., seedlings of rye, maize and sunflower) can be inhibited even under ambient levels of UV-B.(11) Some materials used by mankind degrade more quickly with increased UV-B exposure. Many classes of materials may be susceptible and additional research is ongoing.
All areas of the biosphere appear to be affected to some extent, but some areas more so than others. In fact, some of the consequences, if left unchecked, could be disastrous. Marine phytoplankton, a one-celled plant, produces at least as much biomass as all terrestrial ecosystems combined. It is an essential ingredient in the food chain, as well as being a major oxygen producer and a carbon sink. Recent studies show that the aquatic ecosystem is already under UV-B stress and there is concern that an increase in UV-B radiation will have more detrimental effects.(13) Changes to the quantity of phytoplankton could augment the greenhouse effect. Many larvae, including those of crabs, shrimp and anchovies, would be directly affected because they spend critical periods near the surface of the water, where UV-B can penetrate. Any reduction of the fish population could have an adverse impact on mankind. Fish account for 18% of the animal protein in the world and 40% of the protein consumed in Asia.(14) Both natural vegetation and wildlife are affected. Wildlife, particularly those species that are active during the day, have some of the same problems as human beings. Since the sensitivity of trees and other plant species varies, the diversity and distribution of such species could likely change,(15) or the species could even disappear.(16) Unfortunately, the extent/magnitude of the impact of increased UV-B radiation on vegetation is difficult to determine.
Increases to the level of UV-B also affect microorganisms. A UV-B-induced decrease in microorganisms fixing atmospheric nitrogen would require significant substitution by artificial fertilizer, for example in rice production.(18) In addition, UV-B is a standard laboratory method of causing mutations of microorganisms. Higher natural levels of UV-B could increase the possibility of more rapid mutations, and thus more rapid adaptation to UV-B stress. Many changes could also come to the earth’s atmosphere as a result of a change in the amount of UV-B transmitted through it. The recent UN report on the environmental effects of ozone depletion noted several potential changes related to the air quality in the troposphere.
Another potential long-term effect would be the increased level of carbon dioxide in the atmosphere. The general stunting of vegetation and the reduction in marine phytoplankton would reduce the amount of carbon dioxide removed from the atmosphere and might accelerate global warming.(20) There has been a great deal of publicity about the destruction associated with the depletion of the stratospheric ozone layer. Many of the figures shown to the general public reflect possible impacts of relatively large reductions of the ozone layer. Other factors that may partially offset the reduced ozone levels are rarely discussed. Overall, the current situation is frequently portrayed in the worst possible light, while in fact it is less threatening. Canadians were unduly alarmed in early 1992 by NASA’s preliminary results of the chlorine levels in the atmosphere over the Arctic. These findings, based on NASA’s ER-2 flight of 15 January 1992, showed a chlorine rate of 1.5 parts per billion. This high level of chlorine led to a strong concern that Canada might have had its first "ozone hole," with reductions in the 30% to 40% range, in the spring of 1992. If this had happened in the northern hemisphere, the hole would have approached the 50% range recorded for the well-known Antarctic hole. Later information indicated that the concentration of chlorine was only 0.5 parts per billion, a finding that made unlikely the formation of a "hole" in the spring of 1992. The favourable weather conditions may have helped reduce the impact of ozone depletion, since it is known that extreme cold can accelerate the process of destruction. The actual ozone figures extracted from the Ozone Watch for 11 March 1992 showed that the ozone layer over Western Canada was 15% less than that recorded during 1960-1980, while for the remainder of Canada it was about 5% less.(21) Several factors can offset the increased chlorine levels found in the stratosphere or the reduced level of stratospheric ozone. Increased atmospheric concentrations of CFCs and N2O are predicted to decrease the column content of ozone, whereas increased concentrations of CO2 and CH4 are predicted to increase it. Therefore, it can be seen that the effects of increasing concentrations of CFCs and N2O are to some degree offset by increasing concentrations of CO2 and CH4.(22) Both the tropospheric ozone and aerosols may be masking the consequences of stratospheric ozone depletion by filtering out UV-B in some industrialized regions.(23) Clouds are also thought to filter out UV-B, but no reliable estimates of the direction or magnitude of their effects on UV-B have been determined.(24) Many other factors can also influence the level of ozone. These range from volcanic activity, which dumps gases and aerosols in the atmosphere, to the variable and cyclic output of the sun. Major weather patterns can also be extremely important since they can distribute the ozone more evenly. To help place the current extent of the problem in perspective, some more recently learned facts are listed below:
Research is underway to assess the overall annual effect. While we can do little to stop volcanic action or to change weather patterns, we can reduce man-made products that are helping to destroy the ozone layer. Concern about the ozone layer first surfaced in the 1970s. In the mid-1970s, attention shifted from advanced aircraft to the ordinary aerosol spray can as the primary cause of the ozone depletion. The group of industrial chemicals known as CFCs were then used as propellant; thousands of tonnes of CFCs were being released directly into the lower atmosphere where they began their gradual drift upward to destroy the stratospheric ozone. Since then, the list of materials known to destroy the stratospheric ozone has greatly expanded, although CFCs remain the primary problem. CFCs, and the other ozone-destroying agents, are ubiquitous in almost every society. They are used in a wide range of products that are frequently not recognized as containing ozone-destroying materials. Because of the range of materials involved, the term "ozone-depleting potential" (ODP) was adopted to indicate the ozone-depletion potential of a substance, on a mass-per-kilogram basis, as compared to chlorofluorocarbon-11 (CFC-11). Such a factor is based upon the substance’s atmospheric lifetime, the molecular weight of bromine and chlorine, the substance’s ability to be photolytically disassociated, and upon other factors determined to be an accurate measurement of the relative ozone-depletion potential. Table 1 lists most ozone-destroying agents with their ODPs, as well as some new replacements for CFCs. The hydrochlorofluorocarbons (HCFCs) have a low ODP, while the hydrofluorocarbons (HFCs) have a zero ODP. Both these alternatives to CFCs are "greenhouse gases." This table also shows how ongoing research has caused some of the ODP values to change over time. The biggest change was in Halon H-1301, whose ODP increased from 10 to 16. Table 1
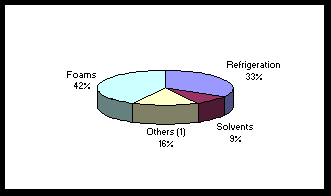
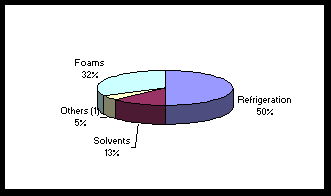
1 These values are based on a new semi-empirical, observation-based method of calculating ODPs, which has better quantified the role of polar processes in this index. 2 These values are more uncertain than those for the chlorine-containing chemicals. Source: Extracted from the Synthesis of the Reports of the Ozone Scientific Assessment Panel, Environmental Effects Assessment Panel, Technology and Economic Assessment Panel, Prepared by the Assessment Chairs for the Parties to the Montreal Protocol, November 1991, p. 4. Each of the main groups of agents has different primary applications. Quantitatively, CFCs are the main ozone-destroying agents. Because of their stable, non-toxic, non-flammable and non-corrosive characteristics, they are used for a variety of purposes and particularly in refrigeration, in the production of foams, in aerosols and in solvents. The other major group of ozone-destroying agents are the halons. Halons are used as fair-fighting chemicals for numerous reasons, including their low toxicity, unobstructed visibility during discharge, low corrosive or abrasive residue, and high effectiveness per pound. Since halons are normally used in closed systems, their major discharge into the atmosphere is during training and systems maintenance tests. Overall, their use is very restricted. The remaining agents, such as carbon tetrachloride (CCl4), are used in several ways, for example, as feedstock to produce CFCs, in pesticides and in dry cleaning agents. Canada’s contribution to global ozone depletion is less than 2%, but its usage pattern is similar to that of many developed nations. Figures 1 and 2 list the percentage usage of CFCs in Canada in 1986 and 1990 respectively. The actual usage was much lower in 1990, 13,700 versus 20,700 tonnes, and this downward trend is continuing. A comparison of the figures also highlights the great increase in the proportion of the usage associated with refrigeration, up to 50% from 33%. Vast amounts of CFCs are also currently held in refrigeration and air conditioning units. B. Technological Alternatives to the Agents The need to reduce the use of ozone-destroying agents has stimulated many technological innovations and use of alternative materials for each major use. The largest and most difficult changes have taken place in refrigeration and air conditioning. In this area and with the halons used for fire protection, the urgent need for change has made recovery, emission reductions and recycling indispensable. In addition to major improvements to recycling and emission, most of the developments for refrigeration and air conditioning have been aimed at finding alternatives to CFC as a refrigerant. Many of these to date have been based on HCFCs and to some extent on HFCs. The first use of HFC-134a in automobile air conditioners was in 1991, but its use is quickly expanding. One of the problems associated with the use of HCFCs and HFCs is that they reduce energy efficiency. Figure 1 (1) Includes aerosols sectors. Source: Environment Canada. Figure 2
(1) Includes aerosols sectors. Source: Environment Canada. The most exciting advance towards resolving the refrigeration and air conditioning problems is the development of the thermoacoustic refrigerator, which has no moving parts and uses environmentally benign gases. While the principle on which it works is simple, the actual design is complex. A loudspeaker at the end of a tube produces an extremely loud note that resonates inside a gas-filled tube. This generates a "standing wave," which effectively transmits the heat from its thinner region towards its thickest bulge, called the antinode.(32) Other possibilities are being tested. The production of foams for uses ranging from insulation to foam cups remains a major use of CFCs. The primary solutions to date have been to lessen the amount of CFCs required or to replace the CFCs with less damaging HCFCs. A new and innovative technique, which uses oxygen and carbon dioxide as a foaming agent, has been developed by the Lily Cup Inc. of Toronto after many years of research.(33) Hydrocarbons have been generally used to replace CFCs in aerosols. A new dispenser that does not use a spray has been developed for asthma users by the Astra Pharma Inc. of Mississauga.(34) One of the principal users of CFC-based solvents was the electronics industry. For most applications, new solvents that do not use CFCs have been developed. One of these is a water-based cleaner containing common citric acid. In 1991, Northern Telecom developed and implemented an alternative technology for soldering electronic circuit boards without using any solvents.(35) Several alternative methods are being investigated to replace halon in fire protection. Until major developments take place, effective halon management can satisfy halon needs for decades. ACTION TAKEN TO REDUCE THE DESTRUCTIVE AGENTS The international community has taken up the challenge of halting the destruction of the stratospheric ozone layer. This firm determination was one of the main reasons for the rapid technological development of alternatives to CFCs. Canada, a very active participant in international discussions, has taken action. In March 1980, this country prohibited the use of CFCs in most common consumer aerosols, such as hairsprays, deodorants and antiperspirants. Canada was the first nation of the 22 countries to ratify the Vienna Convention for the Protection of the Ozone Layer in June 1986. Since this first major international agreement, more comprehensive initiatives to save the stratospheric ozone layer have been agreed to. These international agreements are then translated into national objectives. In Canada, the federal government has been working very co-operatively with the provinces to implement the necessary changes. Industry has also become increasingly involved. Generally, the aim has been to recycle, replace and reduce or destroy the main ozone-destroying agents. In an unprecedented demonstration of global cooperation, the world’s nations committed themselves in 1987 to stringent action to protect the ozone layer. Their major agreement, known as the Montreal Protocol, was designed to reduce and eventually eliminate the use of CFCs. It was signed 16 September 1987 and came into effect 1 January 1989. Even as the Montreal Protocol was being signed, it was recognized that it needed major improvements; these were effected at the London conference in 1990. The Protocol’s list of controlled substance was expanded to include methyl chloroform and carbon tetrachloride. The amendment included the provision of a fund to assist developing nations. At this conference, 13 countries, including Canada, committed themselves to the elimination of CFCs by 1997, three years ahead of the international target. A $240-million fund headquartered in Canada will assist developing nations to switch to more ozone-friendly substances and technology by providing financial assistance, training and technological information. Some of the countries, including Canada, which has a $15-million commitment over three years, have paid their scheduled amounts to the fund; however, as of March 1992, many nations, including both France and the United Kingdom, had not yet done so. The protocol appears to be having the desired effect. Worldwide CFC consumption is now 40% below 1986 levels. At this rate, the objective of 50% reduction will be reached during 1992, three years ahead of the amended Protocol.(36) Since the London amendment to the Montreal Protocol was signed in 1990, many new developments have been announced to accelerate the phase-out of CFCs. These announcements have been made by many of the major industrial nations and by some of the major CFC-related industries. The U.S. has announced that it will phase out the production of CFCs and most ozone-depleting substances by 31 December 1995, rather than by the year 2000. Japan is working on a plan to accelerate the phasing-out of CFCs. Germany, Denmark and the Netherlands are committed to phasing out CFCs by January 1995 and the remainder of the European Economic Community (EEC) will be considering similar action. Canada has announced that it will phase out CFCs by 31 December 1995 rather than the previously announced 1997. Non-recoverable HCFCs will be phased out by 2010 and all production and importation of HCFCs will be ended by 2020. Industry is also responding to the need for change. DuPont, the world’s largest producer of CFCs, will halt its production by 1997, three years earlier than originally planned. Large electronic manufacturers, such as Matsushita, NEC and Sony, all have programs to eliminate the use of CFCs by 1995. As stated earlier in this paper, Northern Telecom has developed a soldering technique that does not need any cleaning solvents. B. Canadian Federal-Provincial Action The fulfilment of Canada’s international commitments requires governments at all levels to cooperate in making the necessary changes to reduce and eliminate ozone-damaging agents. The Canadian Council of Ministers of the Environment (CCME) directed in April 1989 that the Federal-Provincial Action Committee (FPAC) of the Canadian Environmental Protection Act coordinate the development of controls across all jurisdictions. The CCME has taken the lead in organizing multi-jurisdictional participation in the reduction, recovery and recycling of CFCs. On 21 August 1990, the CCME established a working group to develop a National Action Plan for reduction, recovery and recycling. The draft National Action Plan is to be presented formally to the CCME in May 1992. The rapid development of such a major plan demonstrates the level of cooperation between the various governments on this important environmental issue. This plan identifies six major tasks:
Some governments have already started implementing certain aspects of the draft National Action Plan. The recovery and recycling of CFCs for automobile air conditioning during servicing or before vehicle disposal is mandatory in Nova Scotia and Ontario. Similar legislation is expected to be in place in all provinces by the end of 1992. In addition to the work being done directly in collaboration with the provinces, the federal government is also working on other initiatives. On 25 August 1991, the Environment Minister announced that $25 million in Green Plan funding would be used to strengthen Canada’s fight against ozone layer depletion. Of these funds, $9.2 million is to step up the phase-out of ozone-depleting substances (support for controls, regulations, recycling and recovery). The balance of $15.8 million is to be administered by the Atmospheric Environment Service for research, monitoring, ozone impact prediction, and a UV-B warning system. To permit Canadians to take suitable precautions against increased UV-B levels, Environment Canada announced a new advisory service shortly after NASA had confirmed the strong possibility of a substantial reduction of the ozone layer over a large area of Canada. The service commenced 13 March 1992, much sooner than originally planned, and provides daily information on guarding against exposure to the sun in view of changing UV-B levels. A similar service has been in operation in Australia for several years. The developed nations have taken aim at reducing their sources of ozone-damaging agents. Major practical problems have yet to be overcome with respect to the vast depots of equipment and materials containing CFCs and the aspirations of developing nations. Developed nations have been using CFCs since these were invented over 50 years ago. Much of the foam and insulation containing CFCs are in refuse piles and the liberation of their CFCs is uncertain. In addition, the world has a billion refrigeration and air conditioning units, many of which are either poorly maintained and leaking CFCs, and which, when they are no longer serviceable, are simply thrown into junkyards, where the CFCs will eventually escape. No international agreement is in place to ensure that these vast quantities of CFCs will not find their way into the atmosphere. In the longer term, as CFCs are no longer required for recycling, new technologies will be required to destroy them effectively and economically. Both eastern Europe and large nations in Asia such as China and India have problems dealing with ozone-related issues, especially as their ability to change to more sophisticated technologies is relatively limited. Although India and China currently contribute only 3% of the world’s ozone-depleting chemicals, potential growth is enormous. China alone currently produces over eight million refrigerators annually. The $240-million fund established to assist developing nations can provide only a minimum of help, considering the scale of the problem. Canada and other developed nations must have something tangible to offer developing nations, particularly established recovery/recycling mechanisms and economical alternatives to CFCs. Negotiation of "global bargains" will be essential to ensure a truly global solution. Until "global bargains" have been struck, developed nations could consider linking trade development programs and foreign aid policies to the phase-out of CFCs. Even with all the reduction and changes that mankind is now bringing to the production of CFCs, the ozone level will not return to normal for up to 100 years. Even if the control measures of the amended Montreal Protocol (London, 1990) were to be fully implemented, the stratospheric chlorine would peak at about 4.1 parts per billion by the year 2000, resulting in a reduction in ozone of 10% in winter and 5-10% in summer.(37) With this likely long-term reduction in the level of ozone, humanity must adapt to the changing conditions. We can do many things to reduce health risks such as applying a sunscreen with a sun protection factor of at least 15. In New Zealand, school children are urged to wear hats and to eat their lunches in the shade. Wearing high-quality sunglasses treated to absorb UV radiation when outdoors in bright sunlight can effectively protect the eyes. With proper precautions, the negative health effects of ozone reduction can be minimized. Life on earth remains a delicate balance of many different elements. Mankind’s use of CFCs has seriously depleted the ozone layer, which in many respects acts as the earth’s sunscreen. This depletion of the stratospheric ozone has allowed the harmful effects of increased UV-B to worsen so that the UV-B-related destruction is likely to increase until at least the year 2000. The international community has cooperated as never before to remedy this situation. The reduction, recycling and replacement of ozone-destroying agents is progressing. New technologies and replacements are being developed so that ozone-depleting agents can be eliminated. Since 1986, the global usage of CFCs has been reduced by over 40%. Current projections indicate that, in the best case scenario, the ozone layer will be at its worst in about the year 2000 and that it will take many years for it to return to its previous level. Humanity will need to make some changes to adapt to the increased levels of UV-B in order to minimize health problems. It is to be hoped that other life forms on the planet can also successfully adapt to higher amounts of UV-B. Even with full international cooperation, some important issues need to be addressed. How can the existing holdings of CFCs be adequately controlled? How will the developing countries, with their many conflicting requirements, transform themselves into ozone-friendly nations? Until all these issues are resolved, the long-term success of restoring the ozone layer remains clouded. Anderson, Stephen O. "Halon and Stratospheric Ozone Issue." Fire Journal, Vol. 81, No. 3, May-June 1987. "Closing in on Arctic Ozone." New Scientist, 9 November 1991. "Code of Practice for the Reduction of CFC Emission from Refrigeration and Air Conditioning Systems." Canadian Environmental Protection Act, March 1991. Deadly Releases of CFC. HCSC on Environment. Report. June 1990. Environment Canada. Ozone Layer Protection. March 1988. Environmental Effects of Ozone Depletion: 1991 Update. UN Environment Programme (UNEP), November 1991. Milko, Robert. Ozone Depletion and the Montreal Protocol. Library of Parliament, 28 September 1997. "The Stratospheric Ozone Layer over the U.S. Has Been Found Thinner in Summer." Environmental Science Technology, Vol. 26, No. 1, 1992. Synthesis of the Reports of the Ozone Scientific Assessment Panel, Environmental Effects Assessment Panel, Technology and Economic Assessment Panel, Prepared by the Assessment Chairs for the Parties to the Montreal Protocol, November 1991. "Volcanic Dust Threatens the Ozone Layer." New Scientist, 7 September 1991. Watson, Robert. Present State of Knowledge of the Ozone Layer. NASA, June 1988.
(1) Environment Canada, Ozone Layer Protection, March 1988, p. 5. (2) Robert Watson, Present State of Knowledge of the Ozone Layer, NASA, June 1988, p. 3. (3) A "heterogenous" reaction is a reaction involving both gases and solid particles. (4) "Volcanic Dust Threatens the Ozone Layer," New Scientist, 7 September 1991, p. 27. (5) Environmental Effects of Ozone Depletion: 1991 Update, UN Environment Programme (UNEP), November 1991. (6) Ibid. (7) Ibid. (8) Dr. Howard Koh, New England Journal of Medicine, Vol. 325, No. 3, 18 July 1991, p. 171. (9) Ibid. (10) Environment Canada Fact Sheet, p. 1. (11) Environmental Effects of Ozone Depletion: 1991 Update (1991), p. iii. (12) Ibid., p. iv. (13) Ibid. (14) Stephen O. Anderson, "Halons and the Stratospheric Ozone Issue," Fire Journal, Vol. 8, No. 3, May-June 1987. (15) Environmental Effects of Ozone Depletion: 1991 Update (1991), p. iv. (16) Synthesis of the Reports of the Ozone Scientific Assessment Panel, Environmental Effects Assessment Panel, Technology and Economic Assessment Panel, Prepared by the Assessment Chairs for the Parties to the Montreal Protocol, November 1991, p. 6. (17) Environmental Effects of Ozone Depletion: 1991 Update (1991), p. iii. (18) Ibid., p. iv. (19) Ibid. (20) Synthesis of the Assessment Reports… (1991), p. 6. (21) Environment Canada, Ozone Watch, 11 March 1992. (22) Watson (1988), p. 3. (23) Environmental Effects of Ozone Depletion: 1991 Update (1991), p. iii. (24) Ibid. (25) Synthesis of the Assessment Reports… (1991), p. 3. (26) "The Stratospheric Ozone Layer Over the U.S. Has Been Found Thinner in Summer," Environmental Science Technology, Vol. 26, No. 1, 1992. (27) Report, Ozone Scientific Assessment Panel, 1991, p. 3. (28) Ibid. (29) Ibid. (30) "The Stratospheric Ozone Layer…" (1992). (31) Ibid. (32) "Cooling with Sound: An Effort to Save Ozone Shield," New York Times, 25 February 1992. (33) William Murray, "New Technology Developments as a Result of CFC Phase-Out," Prepared for the House of Commons Standing Committee on Environment, Library of Parliament, 1 April 1992, p. 2. (34) Ibid. (35) "No More Ozone-Depleting Solvents," Toronto Star, 16 December 1991. (36) Synthesis of the Assessment Reports… (1991), p. 7. (37) Ibid., p. 5. |
||||||||||||||